
-> our publications in this field.
Strong adverse effects on the healthy tissue in the vicinity of a tumour are a major drawback in current cancer therapies. One innovative technological approach to solve this problem focuses on therapies at the cellular level by applying intracellular probes, i.e. the transfer of nano-sized biocompatible devices into the cells. These devices (particles) must meet the demands of targeted investigation of relevant cell parameters as well as manipulation of the cells. When the transferred nanoparticles exhibit ferromagnetic properties, external magnetic fields can be used for manipulation in deep layers of (human) tissue since they provide a unique way to penetrate tissue non-invasively and without known adverse effects. External static magnetic fields fix the ferromagnetic nanoparticles at a precise position; gradient fields move them and alternating (AC) fields lead to local heating. The latter can be utilized for so-called ?hyperthermia?, i.e. a therapeutic anti-cancer treatment to raise the temperature of tumor tissue in-vivo. This method applies the fact that a cancer cell-killing effect is caused when a temperature above 42?C is maintained in the target volume.
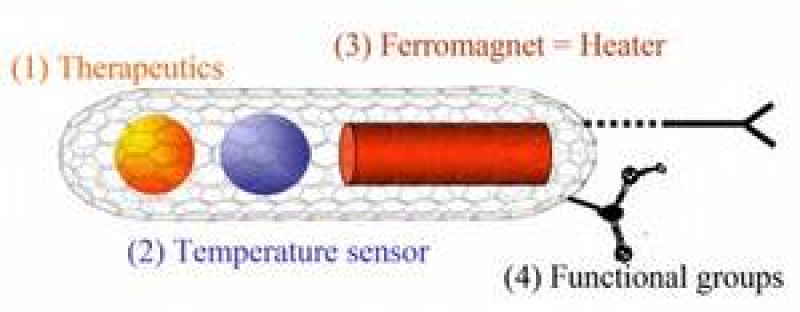
The long-term objective of the research programme is to exploit the potential of carbon-encapsulatedmagnets such as filled carbon nanotubes for human medical applications - with a focus on anti-tumour treatment - which allow targeted release of heat and/or drugs in cancer cells. The nano-devices will act as magnetic nano-heaters, drug-carrier systems and temperature sensors for therapy and diagnosis at the cellular level. To be specific, we:
The usage of carbon-wrapped nanocontainers has several advantages, particularly, in medical applications. First, due to the protecting carbon shell, the number of materials which can be used for applications without toxic adverse effects strongly increases. Second, the outer shell of CNT can be chemically modified, e.g., with cancer-specific binding agents in order to enable attachment to a given tissue. Functionalized CNT can cross the cell membrane and accumulate in the cytoplasm or reach the nucleus without being toxic for the cell. Moreover, a possibility to functionalize the outer surface of CNT allows to control its solubility in physiological solutions not altering physical and chemical properties of encapsulated material. It was demonstrated that stable complex formation of CNT and cationic lipid may accelerate the delivery of nanotubes into bladder cancer human cells. Finally, the container feature of CNT allows simultaneous filling of CNT with a temperature sensor and another probe such as a ferromagnet (=heater), thereby combining different functionalities in one kind of CNT.